FDA approves Actavis intrauterine device to prevent pregnancy
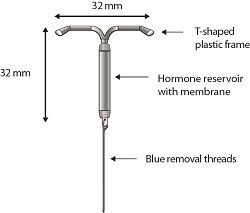
2015-03-12Actavis ($ACT) announced FDA approval of its drug-delivering intrauterine device, Liletta, as a form of reversible contraception. The T-shaped device delivers the drug levonorgestrel, and its dimensions are less than half than those of a standard playing card. Liletta is approved for use by women to prevent pregnancy for up to three years. The therapy's launch is slated for the second quarter of this year, Actavis said in a release.