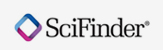Novel New Drugs 2014
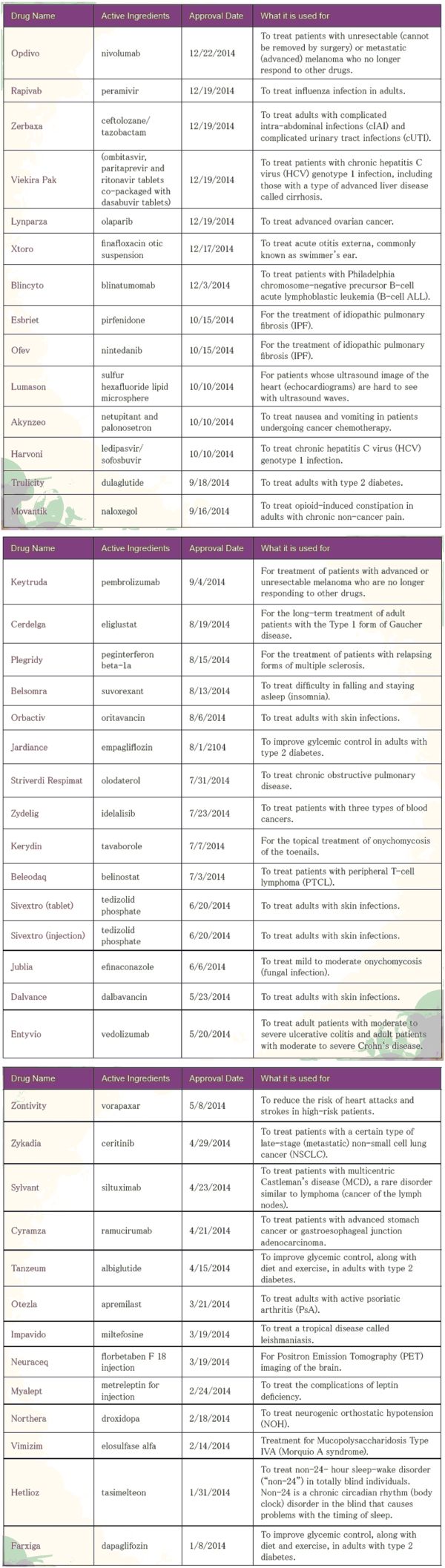
2015-03-17In calendar year 2014, FDA’s Center for Drug Evaluation and Research (CDER) approved 41 novel new drugs, approved as new molecular entities (NMEs) under New Drug Applications (NDAs) or as new therapeutic biologics under Biologics License Applications (BLAs). Below lists CDER’s novel new drugs of 2014.
(http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DrugInnovation/UCM430299.pdf)
Drugs are listed by the date of approval in 2014, backward from the most recently approved.